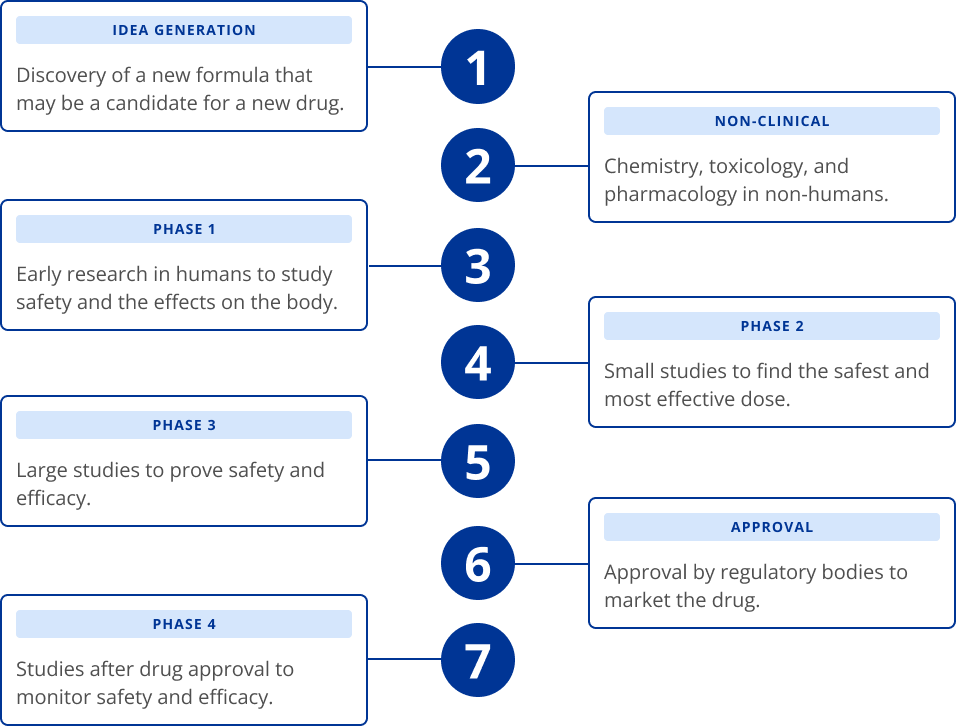
About Clinical Trials
At Alcon we recognize the value of the contributions from research participants and appreciate your partnership.
We understand the importance of scientifically-based diversity across age, ethnicity, gender and race in our clinical trials, and we are committed to designing and conducting clinical trials accordingly.
You can learn more about our clinical trials by asking your eye care provider about studies or by visiting:
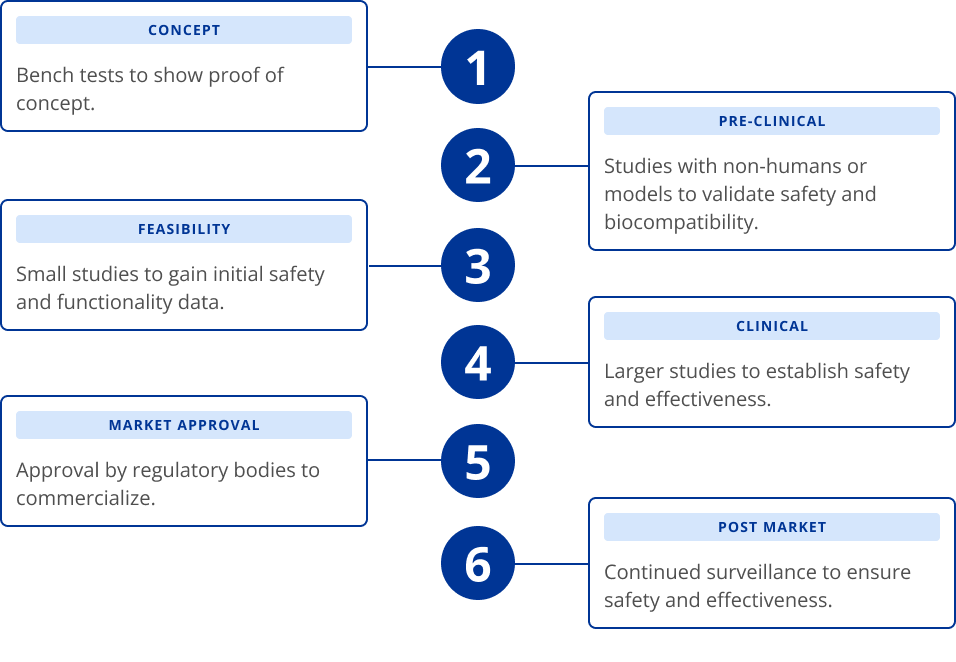
Medical Device Development Process
Every medical device goes through a rigorous process from concept to market. This is to ensure that when it reaches the market, the medical device is safe and effective for consumer use.
The medical device development process can take years to complete from the time an idea is born to the approval of regulatory bodies to commercialize the device.
In general, Alcon's medical devices follow the process outlined below.