
Brilliant Perspectives: Paul Hallen on how Alcon’s Surgical Innovations Are Elevating Visual Outcomes in Vitreoretinal Procedures
July 30, 2025
time to read 5 minutes


July 30, 2025
time to read 5 minutes

Paul Hallen, Vice President and Distinguished Fellow, Surgical Innovation, at Alcon, has played a large role in shaping the trajectory of Retina at Alcon. Following an unprecedented year of new vitreoretinal technology from Alcon, we spoke with Paul about how far the industry has come, and where Alcon—and the evolving landscape of vitreoretinal surgery—go from here.
Let’s start at the beginning. When did Alcon start investing in retinal surgery, and where are we now?
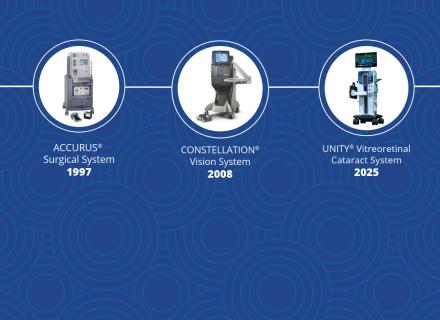
Since Alcon entered the retina space in 1997, we’ve helped lead a steady cadence of progress. Starting with the ACCURUS® system and progressing through CONSTELLATION®, we have consistently delivered improvements in surgical technology.
For example, take vitrectomy cutting speeds. In ‘97, the maximum cuts per minute (cpm) we offered was 600. By 2019, we evolved that to 20,000 with CONSTELLATION, and now to 30,000 with the UNITY® Vitreoretinal Cataract System (VCS). These consistent advancements have made vitreoretinal procedures more efficient and precise. In fact, a clinical study comparing 20,000 cpm and 10,000 cpm cutters in epiretinal membrane surgery found that the higher-speed cutter significantly reduced vitrectomy time, demonstrating safety and efficiency in vitreous removal.1
During that time, how has Alcon turned surgical challenges into technological progress?
One of the key challenges surgeons have faced over the years is limited visualization during procedures. Traditional microscopes can be limited by depth of field, which creates hurdles for surgeons performing procedures that require focused and accurate maneuvers.2
As the global leader in eye care, we’re agnostic as to where innovations that solve problems like this come from. If there is a technology on the market capable of solving these challenges, our Business, Development and Licensing (BD&L) team works hard to see if it could be a fit for our existing portfolio and if the product can scale to allow for more accessibility to surgeons around the globe. That is exactly what happened in 2018 when Alcon acquired the NGENUITY® 3D Visualization System, a device providing high-resolution, stereoscopic visualization that enhances depth, contrast and precision in the operating room (OR).3
Grieshaber, Alcon’s R&D site in Schaffhausen, has been central to this evolution. Recently, marking its 110th anniversary, the associates who have been part of the site’s century-long history have helped transition the industry from 20-gauge tools to today's minimally invasive micro-surgical instrumentation. Built on a legacy of precision engineering and trusted, high-quality products, the team at this facility produces more than 1.4 million hand-crafted ophthalmic instruments and accessories annually.4 The site’s recent expansion into Neuhausen am Rheinfall reaffirms Alcon’s commitment to pushing surgical innovation forward.
What about solutions developed in-house?
Sure—I’m very proud of the challenges our R&D team has solved with innovations we’ve built from the ground up—particularly the risk of incorrect gas mixes, which can lead to complications. To put the issue in perspective: in a 2023 global survey of 906 retina specialists, approximately 50% of surgeons reported seeing at least one patient who received an incorrect gas mix during surgery. Of those, approximately half (roughly 25%) noted that the error resulted in vision loss or other permanent complications.5*
That’s exactly what led us to develop the UNIFEYE™ and UNIPEXY™ systems. With an initial introduction to the U.S. market in 2025, both are designed to take the guesswork out of the process—delivering pre-mixed, predictable gas-to-air ratios so surgeons can operate with more confidence and consistency.
By simplifying everything from storage to delivery, UNIFEYE helps make workflows smoother and more efficient in the OR. And thanks to its handheld design, surgeons stay in full control throughout the procedure—supporting both safety and precision when it matters most.
How is Alcon expanding on vitreoretinal innovations like UNIFEYE and UNIPEXY?
We’re taking the next step in vitreoretinal innovation with the UNITY® Vitreoretinal Cataract System (VCS)—a combined platform designed for superior efficiency.6-9,**The system features multiple technologies that our industry has never seen before, including a high-speed vitrectomy probe that delivers up to 30,000 cuts per minute with a continuously open port. That means efficient vitreous removal designed to minimize turbulence, helping for more stable procedures. It’s also the first system to introduce a multi-spot laser probe that can dramatically reduce laser application time, supporting the efficiency needs of the current OR.
To support consistency throughout surgery, UNITY VCS pairs advanced fluidics with customizable red, green and blue (RGB) lighting for stable intraoperative pressure and clear visibility. We've also designed the 27 + DS System Consumables to deliver the stiffness of 25+ instruments or better, optimizing control and reach to support performance.8,10
UNITY VCS also pairs with UNIFEYE—bringing together two powerful technologies in one system. It’s all part of our focus on helping surgeons do their best work, every time.
What do these innovations mean for patients and patient safety?
Each advancement is designed with the goal of minimizing impact to the patient’s eye, supporting safety and consistent outcomes. For example, using smaller, stiffer gauge sizes can help reduce invasiveness while still offering the control surgeons need for delicate retinal maneuvers.
At the same time, tools like UNIFEYE help eliminate manual steps and reduce human error. By delivering pre-mixed, predictable gas-to-air ratios, surgeons can operate with added confidence. It’s all part of an effort to simplify procedures, mitigate risk and offer the highest standard of care to patients.
Where does Retina R&D at Alcon go from here?
Our investment in Alcon’s R&D engine continues to pave the way for future technologies designed to address both existing challenges and unmet needs in retina care. From advanced work in membrane technologies to explorations in digital visualization and AI-driven initiatives, our product pipeline is robust.
What would you say to surgeons who haven’t tried Alcon’s retina portfolio, or are hesitant to make the switch?
Alcon remains committed to investing in solutions that drive meaningful progress in retinal care. With an industry-leading breadth of surgical products and solutions in eye care, Alcon is uniquely positioned to meet the diverse and evolving needs of patients and surgeons around the world. We engage closely with our customers to understand the real-world challenges they face in the OR and clinic, and design solutions that help deliver the best possible patient outcomes.
Through Alcon-Initiated Trials (AITs) and Investigator-Initiated Trials (IITs), we are building a growing body of clinical evidence that demonstrates the safety, efficacy and efficiency of our vitreoretinal technologies. These studies not only validate current performance but also help inform future product enhancements and best practices. From the OR to our R&D facilities, the goal is to empower surgeons with tools that deliver excellent outcomes, reduce procedural complexity and minimize impact on the eye, ensuring patients receive the best possible care. We’re looking forward to sharing results like this for our latest vitreoretinal innovations in the coming months.
*Response given to the following question on the ASRS 2023 Preferences and Trends Membership Survey: “Have you seen a patient who had (or was suspected to have) a wrong gas and/or concentration placed in the eye?” n=906.
**Compared to CONSTELLATION and CENTURION Vision Systems. Based on bench data.
References:
© 2025 Alcon Inc. GLB/IMG-UVC-2500046